The Construction Plan of mRNA Therapeutics Manufacturing, Technology Development Facility has been Selected for the METI Subsidy Programs
ARCALIS, Inc. (headquartered in Minamisoma City, Fukushima Prefecture; hereinafter “ARCALIS”) announces that the construction plan of ARCALIS mRNA Therapeutics Manufacturing, Technology Development facility (hereinafter “MD facility”) has been selected for the secondary offering of “Biopharmaceutical Production Base Development Project for Strengthening the Vaccine Production System” by the Ministry of Economy, Trade and Industry. (hereinafter “Project”).
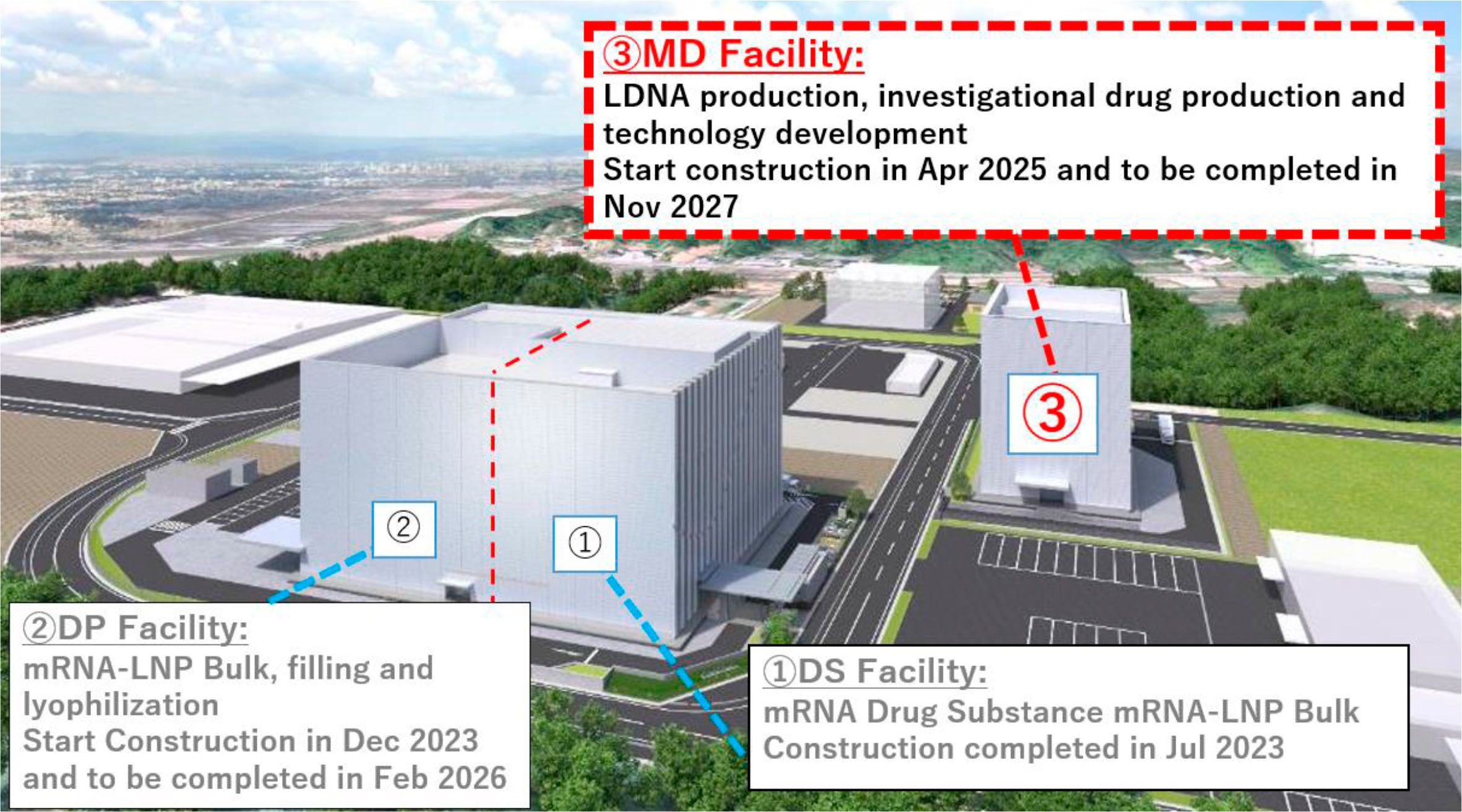
The state-of-the-art MD facility will include facilities to produce template DNA which serves as the blueprint for mRNA, GMP-compliant manufacturing facilities for investigational drugs and small quantities of pharmaceuticals, and equipment to expand research and development capabilities in the future.
In normal times, the MD facility will manufacture template DNA used for the development of various vaccines and therapeutics, investigational drugs, and mRNA pharmaceuticals for personalized medicine, which require high-mix low-volume production. Additionally, in cases where infectious diseases like COVID-19 continue to occur, the MD facility will engage in the pilot production of mRNA vaccines targeting epidemic strains and the manufacturing of prototype vaccines.
Furthermore, in the event of a pandemic, upon confirmation of the genetic sequence of the pandemic virus strain, the MD facility aims to rapidly produce template DNA currently being outsourced to foreign manufacturers, establish the production process within 6 weeks of the pandemic declaration, and commence the shipment of vaccine investigational drugs within 100 days (14 weeks).
Being selected for this Project, ARCALIS will be able to add the manufacturing development capabilities on top of the mRNA drug substance facility (completed in July 2023) and the drug product facility (scheduled for completion in 2026). This will enable ARCALIS to achieve seamless collaboration between manufacturing technology development and production within a single facility, establishing a domestic capability to develop and manufacture mRNA pharmaceuticals consistently and expeditiously.
[Summary of this project]
| Subsidized Projects | mRNA Therapeutics Manufacturing, Technology Development facility (MD facility) construction plan |
|---|---|
| Overview of Normal time and Emergency time Production | <Normal-Operation> |
| Construction Period | Completion of the project by the end of 2026 after the decision to subsidize |
*1 Project for Establishment of Biopharmaceutical Production Facilities to Strengthen Vaccine Production System (Second round of public solicitation)
In order to prepare for infectious diseases that may become a threat in the future, we will support the introduction of facilities to establish bases with dual-use facilities that can manufacture biopharmaceuticals according to the needs of companies during normal times and switch to vaccine production in the event of an infectious disease pandemic, as well as facilities for formulation and filling essential for vaccine production and for the production of materials necessary for the manufacture of pharmaceuticals. In addition, the Ministry of Economy, Trade and Industry (METI) will provide support for the introduction of facilities for vaccine production. For details, please visit the Ministry of Economy, Trade and Industry’s website.
(URL: https://www.meti.go.jp/information/publicoffer/kobo/2023/k230317001.html )
*2 About Fukushima Minamisoma Plant
As a CDMO for mRNA drugs and vaccines, ARCALIS has completed the construction of an API manufacturing plant in Minamisoma City in July 2023 and to start construction of a formulation manufacturing building in December of the same year. Currently, ARCALIS is working to establish a manufacturing system for the API for Replicon Vaccine (a next-generation mRNA vaccine) developed by Arcturus, a major shareholder of ARCALIS.
